eCTD Lifecycle Viewer
Mono eCTD Viewer streamlines review process, reduces response times to Agency requests, and ensures faster approvals for your products.
What is your usage scenario?
You publish eCTD sequences as a service to pharma companies. You need to allow your clients to see the results before final approval. Use Mono eCTD Viewer.
You need to review and check the final result (documents, lifecycle, hyperlinks, attributes, regional metadata ...) before sending to Health Authorities. Use Mono eCTD Viewer.
Trying to use Edge, Chrome or Internet Explorer (IE) to view eCTD backbones?
Internet Explorer is no longer supported and trying to use a Browser to navigate eCTD's XML backbones could be cumbersome. You need a true eCTD navigation and reviewing system.Sequence / Current / Cumulative?
Mono eCTD Viewer was built with the ease of use in mind: the complex XML eCTD (Electronic Common Technical Document) structure of an eCTD lifecycle is presented in a user friendly manner.
Mono eCTD Viewer's fluent user interface hosts a filter pane, a backbone tree (including current, cumulative and custom views) and a set of tabbed panes to quickly preview documents, metadata attributes, keywords (controlled and custom), envelopes (regional information), lifecycle info, STFs, document types, PDF hyperlinks, etc. Can be easily configured to match your (client) needs.
To load a lifecycle, eCTD sequences (backbones, submission units, documents and folders) need to be available through the file system (local drive or network shared drives).
Mono eCTD Viewer's user interface is available in multiple languages (English, Dutch, French, German, Spanish, Italian).
Mono eCTD Viewer can load and process any eCTD submission, as long as sequences conform to ICH and regional eCTD specifications: US (U.S.A.), CA (Canada), EU (Europa + UK), BA (Bosnia and Herzegovina), CH (Switzerland), GCC (Gulf Cooperation Council), JO (Jordan), AU (Australia), TH (Thailand), TN (Tunisia), ZA (South Africa), CN (China), SG (Singapore), UA (Ukraine), WHO (World Health Organization), .... eCTD (both version 3.2.2 and 4.0) submissions.
Get Your Mono eCTD Viewer License
Mono eCTD Viewer is available in four editions: Beginner, Professional, Expert and Master. The Beginner edition (eCTD v3.2.2) is FREE and has most limitations and none of extra tools. The Profesional edition (eCTD v3.2.2) has some features restricted. The Expert edition (eCTD v3.2.2 and eCTD 4.0) includes most of the features and eCTD lifecycle tools. The Master edition (eCTD v3.2.2 and eCTD 4.0) has no restrictions and includes ALL the in-depth features and eCTD lifecycle tools you might need.
Ready to try the fully functional trial? Request your
free Master edition copy.
Ready to unleash all your potentials? Purchase your license:
Need more? Benefit from extra discount (up to 50%) when purchasing more than 5 licenses. Contact Us.
Mono eCTD Viewer Editions
Mono eCTD Viewer is a multi-regional eCTD re-viewing solution packed with lots of powerful features to help you explore and understand eCTD product's lifecycle more easily. Are you a beginner or an eCTD lifecycle master?
Mono eCTD Viewer Features
Mono eCTD Viewer is under constant testing and development: new features, as a result of user requests, are being added daily. Check out some of the more exclusive features you can benefit from:
Your eCTD Viewing Solution
Even if you already have an eCTD viewing solution - try eCTD Viewer to unleash all the potentials of an eCTD lifecycle!
Supports both eCTD version 3.2.2 and eCTD version 4.0.
Using additional tools included like MD5 Fixer, PDF Hyperlink Explorer, Submission Comparer you can understand your e-submissions more deeply.
NeeS / VNeeS / Other Electronic Dossier Formats
While Health Authorities are moving away from NeeS toward eCTD, there are Non-eCTD submissions you need to browse, navigate, view and re-view.
NeeS Viewer handles both (human) NeeS and (veterinary) VNeeS and other electronic dossier formats (e.g. Swissmedic eDok).

eCTD Validation
Makes sure your eCTD sequences are technically valid and compliant with the latest regulatory standards and requirements.
While Mono eCTD Viewer will load and process even invalid sequences - you can run in depth technical validation on any loaded sequence and save the full detailed validation report.
How healthy is your eCTD product? From (ages old) sequence 0000 to today? eCTD Product Validation processes all sequences for a specified eCTD product and performs most important checks related to the validity of content (e.g. documents exist?), lifecycle (e.g. replace operation on deleted leaf?) and checksum matching (e.g. have your documents been modified?).
For more eSubmission validation options visit: Mono eCTD Validator.
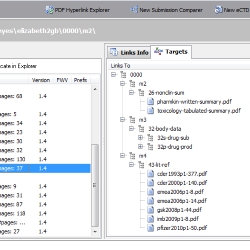
PDF Hyperlink Explorer
Need to explore all hyperlinks between PDF documents sharing a common folder (like an e-submission, eCTD/NeeS sequence folder)? PDF Hyperlink Explorer quickly scans and processes PDF documents and hyperlinks to present a clean and simple view of how your documents are cross-linked. Check for the type of the hyperlink (including link text, page, position), target documents, whether a document is optimized for Fast Web View, if PDF properties are valid (initial view magnification, initial view page layout, PDF version, etc.). Process any PDF collection - not only an eCTD/NeeS submission!
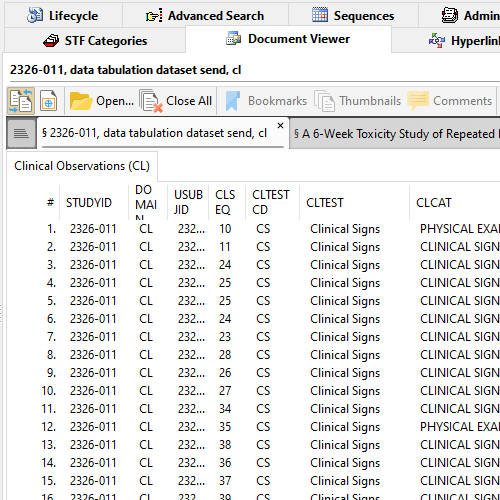
XPT Viewer in Document Viewer
Lots of electronic datatasets in your submission? Having trouble viewing data in XPT (SAS XPORT Transport) files?
XPT Viewer quickly parses XPT datasets and present all Members including all Observations per Member, in a tabular format.
Readily check if the study start date is defined. Make sure StudyID (or SPREFID) matches STF's Study ID.
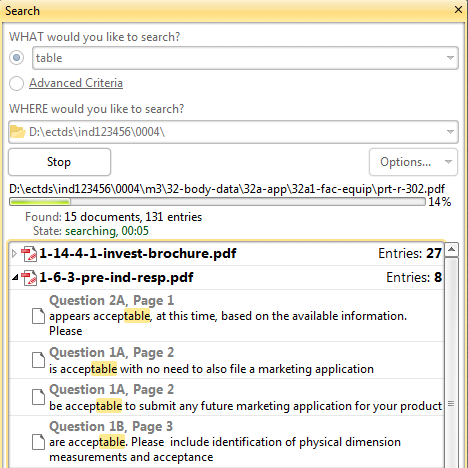
Advanced PDF keyword search in [sequence/product] folder
Need to find all documents having keyword "study-123"? Need to locate all documents having a link to "responses.pdf" Need to locate all mentions of "Table X" in all your sequence documents? And you need it fast?
No problem! The Advanced Search pane in Document Viewer allows complex in-depth keyword searches - to locate all documents matching the search in a selected [sequence or product] folder and all subfolders.
Save search results as a PDF document.
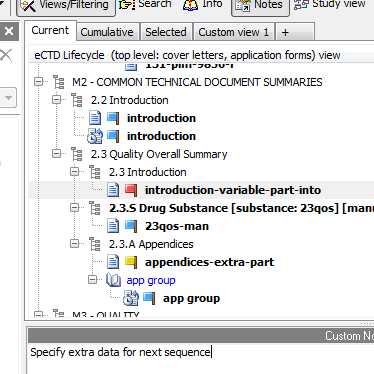
Two-way Experience
We believe an eCTD viewer should not only allow you to simply view documents in an electronic submission lifecycle. How about having an option to add custom notes for every document in your electronic submission? How about being able to also mark a document with a "flag" using custom flag names?
eCTD Viewer lets your transform simple viewing into a more two-way experience. Save (and load) multiple notes per one submission. Share your notes with others in your team!
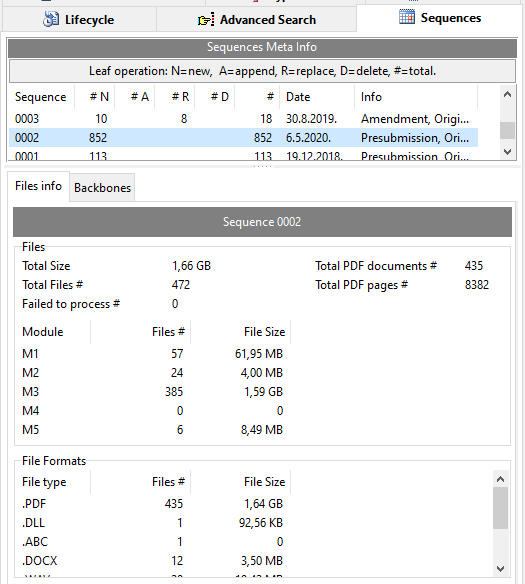
Sequence Files Info
Interested in some meta info on files/documents submitted for a sequence in your eCTD product?
For each sequence get the total number of files, total PDF documents, total PDF pages, total documents (count and size) per module and per file extension.
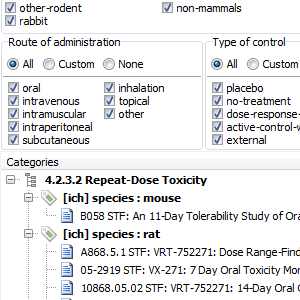
Lots of STFs in your (FDA) eCTD submission?
STF Categories tab will display all your study tagging files grouped using category elements as specified in the STF. This includes: 4.2.3.1 Single dose toxicity (grouped by species and route of administration), 4.2.3.2 Repeat dose toxicity (grouped by species, route of administration, and duration if applicable), 4.2.3.4.1 Long term [carcinogenicity] studies (grouped by species) and 5.3.5.1 Study reports of controlled clinical studies pertinent to the claimed indication (grouped by type of control).
Study View will display documents in a selected STF grouped by their file-tag values.
File reuse / Reference leaves / Virtual documents
The name is not unique but the idea is encouraged by ICH and Agencies: eCTD provides the ability to submit a file once and display it in multiple locations of the eCTD by providing multiple leaf elements referencing that file. File Reuse tab visually displays how your submission documents are reused in a sequence or even across sequences. Reviewers or your product, and you, can readily identify files which are referenced multiple times.
Standard features
- Current / cumulative / sequence / regulatory activity / custom views.
- Clear presentation of submission metadata, such as indication, dosage form, manufacturer, excipient.
- Visual indicators for lifecycle leaf operations (new, append, replace, delete).
- Display of append documents under their "parent" / original document with implicit delete operations.
- Metadata attribute viewer.
- Load a document in embedded PDF viewer, external PDF viewer or just locate in Windows Explorer.
- Save screen layouts (to quickly reconfigure screen UI).
- Basic document info (title, sequence, target) above the preview.
- Compare two sequences (or any two folder structures).
- Leaf flagging (mark a leaf with a different flag).
- Save and re-apply filters: by module, by sequence, by leaf operation, by title, by target sequence, by file name, by leaf ID, ....
- Multilingual user interface (English, Dutch, French, German, Spanish).
- Load lifecycle (from a specific) up to specific sequence.
- Lifecycle report.
- Save regional metadata as CSV.
- Save current view as CSV.
- Save STF/Study View as CSV.
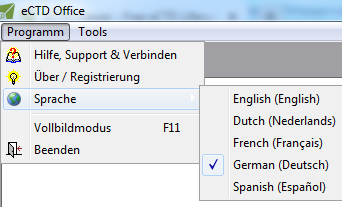
Speaks Your Language!
Not fluent in English? No problem!
Parlez-Vous Francais? Sprechen sie Deutsch? ¿Habla español? Spreekt u Nederlands? Parli italiano?
Have the user interface translated to the language you know in one click and on-the-fly!
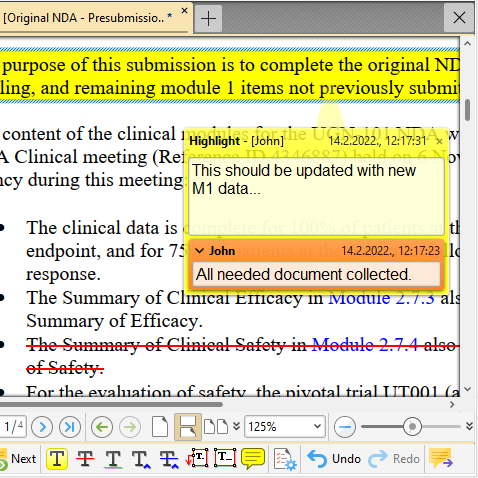
Markup Annotations (/Comments)
Want to comment on sequence documents? How about adding/editing and viewing PDF style markup annotations? Speed up your internal reviewing process using standard PDF markup tools: highlight, insert-text, replace-text, strikeout-text, sticky note, pop-up note, text box. Add replies, set statuses.
Easily summarize all the comments in the document - summary can be presented in various ways.
Don't worry! Markup is stored in a separate file -> your documents (and backbones) are not altered in any way! What's more, you can also markup your non-PDF documents.
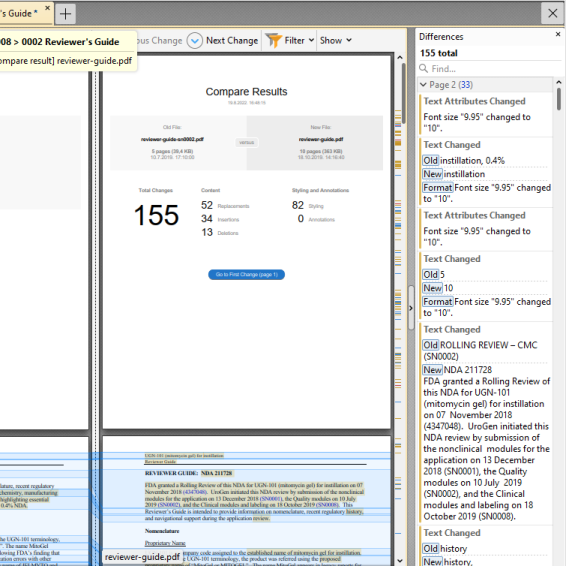
Compare documents
Quickly identify changes by comparing two versions of a submitted document: the lifecycle replacement and the 'original'. Content (text, formatting, etc.) changes in documents are analyzed and a side-by-side comparison document detailing differences is generated. The comparison tool lets you apply filters to see what has been changed: text, formatting, annotations, images or header and footer.
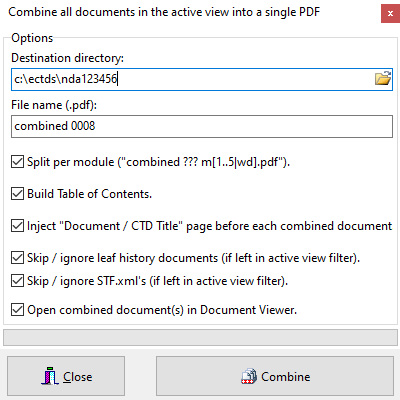
Combine documents
Easily combine (/merge) all documents in the active view (current, cumulative, sequence, custom) into a single (or per Module) PDF document. TOC is created along with bookmarks for easier navigation.
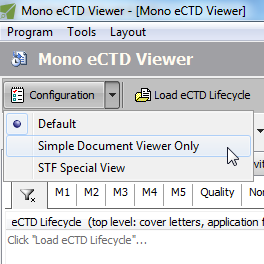
Multiple Configuration Sets
How complex or simple do you want the user interface to be? Are you interested only in documents and their lifecycle? Or, are you only interested in Study Tagging Files (STF)? Would you like to go to the last sequence directly after loading the lifecycle? What's your preference: Current or Cumulative or Sequence view as default? And how do you want them ordered?
What's your role? A RegOps member? Or QC is what you need to do? Want to pretend you are the Reviewer at the Agency?
Have unlimited number of configurations saved - and quickly, on-the-fly, apply to change your needed role.
MD5 Checksum Fixer
You need to do just a little edit to your cover letter or the application form? You noted just one link is missing in your responses document? A few leaf titles would need to be slightly updated? If you do any such change your submission will become invalid! The backbone files (index.xml and cc-regional.xml) store the checksum (MD5) of each document that is a part of your sequence - if you do any changes you will experience the "wrong checksum in index-md5.txt" error with eCTD validators.
Don't worry! The "Backbones MD5 Checksum Fixer" tool will quickly re-scan all your documents and update the "checksum" attribute in backbones files and the index-md5.txt.
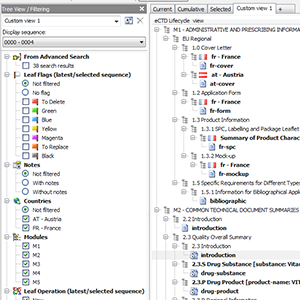
Current, Cumulative? Boring. How about your own Custom Views?
As unique as you are. Current and cumulative views are ok, but when you need more
control you want unlimited number of easily switchable custom tailored (savable) views.
Display only one sequence or all sequences in a regulatory activity.
Have 2.3.S, 2.3.P, 3.2.S, 3.2.P sections (drug substance, drug product, dosage form, manufacturer, indication) merged into and displayed as a single section collecting all documents - to more easily navigate to and compare documents across different branches.
Reviewing an EU (/GCC) MRP / DCP procedure? More countries involved?
Filtering section includes all countries involved in the life of a product and you can quickly hide those you are not interested in momentarily.

Envelope/Regional Elements Analyzer
Why stop your eCTD lifecycle experience at the sequence / document / operation level? Would you like to quickly find out what sequence replaces sequence 0000 with submission type = "amendment" / "supplemental-data"? The "Envelope / Admin Elements Analyzer" allows for complex queries to envelope elements - get the info in just a click.
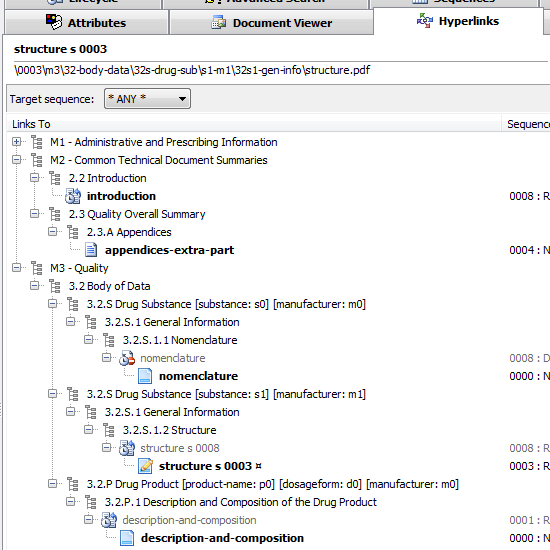
Documents linking to other (same sequence or cross-sequence) documents
You think a study-report.pdf under an M5 section has hyperlinks to a few other documents in the submission? Not sure if this one points to M2 data and if all the hyperlinks are functional? Activate the Hyperlinks tab and find the answers - visually identify targeted documents in the same or across different sequences.


